In the fight against ovarian cancer, one of the biggest challenges is invisibility. The body’s immune system, usually a vigilant defender, often fails to recognize ovarian tumor cells as dangerous. That’s where GW Cancer Center researcher Kevin Nestler, a PhD candidate in the Chiappinelli Lab, steps in. His work explores a fascinating biological puzzle: how subtle edits to RNA help cancer cells hide from immune attack, and how removing those edits could help the immune system fight back.
Alerting the Immune System Through Viral Mimicry

Nestler’s work centers on a process called viral mimicry, a cellular illusion in which cancer cells trick the immune system into thinking they’re infected by a virus. When this happens, the immune system responds by sending in killer T-cells to destroy the “infected” cancer cells.
Here’s how it works: roughly half of the human genome is made up of transposable elements (TEs), ancient, virus-like fragments of DNA that have been passed down for millions of years. Normally, these elements are silent, locked away in the genome like sleeping relics. But certain drugs, known as epigenetic therapies, can reactivate RNA from them. Once awakened, these viral RNAs set off cellular alarm bells and call in immune defenses.
“The goal,” Nestler explains, “is to flip a cold tumor — one that hides from the immune system — into a hot tumor that’s full of immune activity.” In ovarian cancer, which is often “immune cold,” that shift could be life-changing.
RNA Editing: The Writer, Reader, and Eraser
But there’s a catch: many cancer cells already have some TE RNA, yet they still escape detection. Nestler’s research seeks to understand why. The answer, he suspects, lies in chemical modifications to RNA. These tiny molecular marks can alter how genetic information is interpreted.
To make sense of this complex process, Nestler compares it to the editing of a manuscript:
- Writers are enzymes that add chemical marks, like editors making changes to a draft.
- Readers are proteins that interpret those marks, deciding what happens next: should the RNA be translated into protein, degraded, or preserved?
- Erasers are the copyeditors who can remove marks, revising the RNA’s message yet again.
These modifications, taken together, form what scientists call the epitranscriptome, a layer of regulation as nuanced and dynamic as any newsroom.
Nestler focuses on one particular mark, N6-methyladenosine (m6A), which can dramatically change how RNA behaves. “We think the presence of m6A on RNA derived from these transposable elements might actually stop them from forming RNA that causes an immune response,” he explains. “That would prevent the immune system from recognizing and attacking the tumor.” In other words, cancer cells might be “editing” their own manuscripts to silence the alarms before they sound.
Turning Down Cancer’s Defense Mechanisms
If this hypothesis is right, the implications are profound. Nestler’s project tests whether blocking the writers that add m6A marks can help unmask tumors. The drug he’s using to do this is already in phase I clinical trials, meaning it’s being tested in humans for safety.
By combining this m6A inhibitor with existing epigenetic therapies that activate TEs, Nestler hopes to produce a stronger, more sustained immune response against ovarian cancer. “If we can remove the cancer’s ability to hide, we might make other immunotherapies like checkpoint inhibitors more effective,” he says. For a disease that has long resisted immune-based treatments, this represents a promising new strategy.
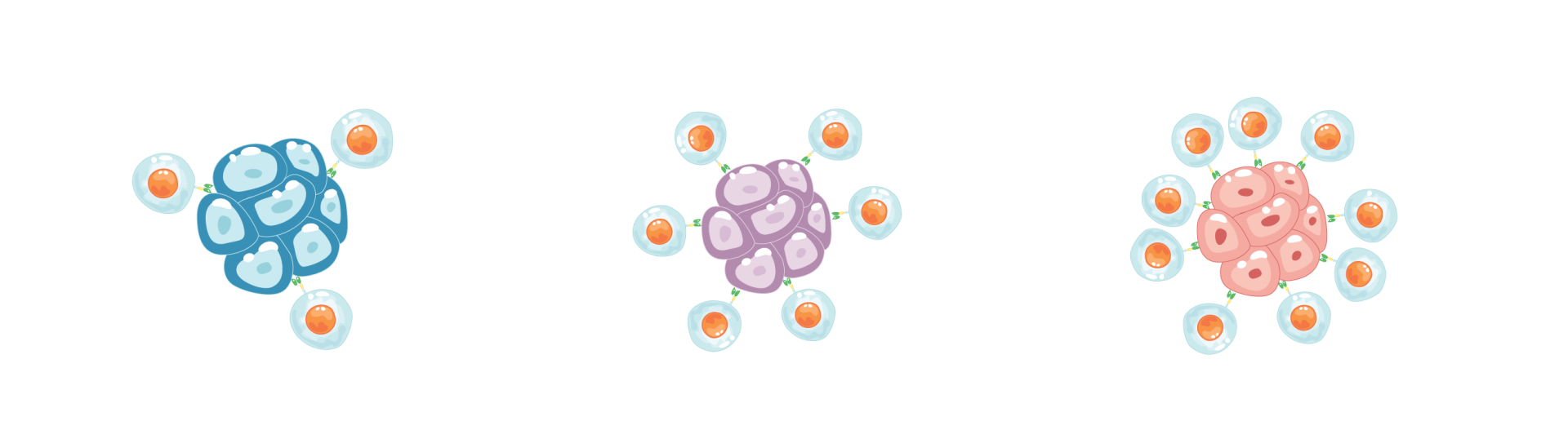
GW Cancer Center researcher Kevin Nestler is testing the hypothesis that epigenetic therapy can help transform a “cold” tumor with little immune activity into a “hot” tumor teeming with immune cells. In his model, treatment awakens the immune system: a cold tumor (left) begins to “warm” (center) as immune cells recognize cancer, eventually becoming a hot tumor (right) with robust immune attack.
Nestler’s project is supported by a competitive Ruth L. Kirschstein Predoctoral Individual National Research Service Award from the National Cancer Institute (NCI), which funds PhD students conducting cancer research. The grant supports his work to uncover how RNA modifications affect immune recognition and to explore novel strategies for making ovarian tumors more visible to the immune system. The two-year award covers a stipend, training-related expenses, and a portion of tuition costs.
A Personal Motivation, Mentorship, and Momentum
For Nestler, the science is deeply personal. “My sister had triple-negative breast cancer, and a close family friend passed away from ovarian cancer,” he shares. “That experience made me want to be part of finding better solutions.”
It’s also a field that’s advancing at lightning speed. “The study of RNA epigenetics is still new, but it’s exploding,” he says. “Every month, new discoveries are showing us how much influence these chemical modifications have. It’s a completely new way to think about targeting cancer.”
Nestler credits his mentor, Kate Chiappinelli, PhD, with helping him turn curiosity into discovery. “Kate has this amazing ability to trust her students,” he says. “She gives you room to explore, to test your own ideas, and she’s always there to guide you when it counts. That kind of mentorship makes all the difference.”
Together, they’re part of a growing wave of scientists redefining how cancer can be treated, not by killing cells directly, but by rewriting the molecular instructions that let cancer hide in plain sight.
In short: Kevin Nestler’s research is about giving the immune system its sight back by erasing the edits cancer uses to stay invisible. It’s not just clever molecular biology; it’s storytelling at the cellular level. And if the story ends the way Nestler hopes, it could mark a new chapter in how we treat ovarian cancer.



