In Greek mythology, a chimera is a creature with a lion’s head, a goat’s body, and a serpent’s tail. In cancer care, Chimeric Antigen Receptor (CAR) T-cell therapy is a cancer immunotherapy procedure where a patient’s T cells are removed from the body and engineered into new and differentiated cells – think cellular-level chimeras – and then infused back into the patient so that the immune system, not chemotherapy, fights their cancer. The George Washington (GW) Cancer Center is making investments and strides in CAR T therapy with the goal of increasing the remission rates of blood cancer patients.
Amarendra Neppalli, MD, Director of the GW Cancer Center Stem Cell Transplantation and Cellular Therapy, explained that CAR T therapy is the most innovative technology in fighting the liquid tumors of leukemia, lymphoma, and multiple myeloma. Soon, CAR T therapy will be an option for blood cancer patients at GW Cancer Center.

For the past 20 years, the GW Cancer Center has been performing autologous stem cell or bone marrow transplants. Bone marrow produces the red blood cells that carry oxygen throughout the body, platelets that help the blood to clot, and infection-fighting white blood cells. Autologous transplants use the patient’s own stem cells, which are collected and stored in a freezer before the patient undergoes high-dose chemotherapy treatments aimed at eradicating cancer cells, that could damage their bone marrow, the spongy center of most bones. After chemotherapy, the stem cells are returned to the body, which will help regenerate the bone marrow.
CAR T-cellular therapy only became available as a cancer treatment approximately seven years ago in 2017. For the past three years, GW has invested in building a Good Manufacturing Practice (GMP) laboratory to process stem cell transplants and to have the capabilities to manufacture CAR T therapies. GMP facilities follow strict quality assurance guidelines and procedures that ensure the consistent production and delivery of safe medical products. Construction of the lab was completed last month, and it is scheduled to open in January 2024.
How does CAR T-cell Therapy Work?
First, the patient undergoes a thorough examination of their organ functions, and the medical team checks for infectious markers. If it’s determined that the patient would tolerate and benefit from the CAR T process, T-cells are collected via a catheter in the arm.
The blood goes into an apheresis machine that then separates it into its four components: red and white cells, platelets, and plasma. The T-cells, or lymphocytes (a type of white blood cell), are directed into a collection bag, and the rest of the blood is returned to the body through a different tube. This process can take up to three to four hours.
Genetic Modification
The extracted T-cells are genetically engineered in the GMP laboratory with the introduction of an engineered gene. The new gene encodes a CAR designed to recognize specific proteins, or antigens, on the surface of cancer cells.
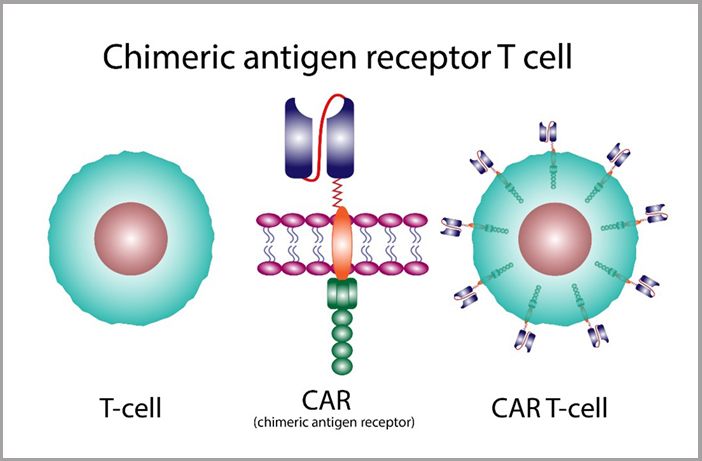
Multiplication and Activation
The modified T-cells are given time, typically up to several weeks, to multiply in the lab, creating a much larger population of these new and specialized CAR T-cells. The new cell population is activated to enhance its cancer-fighting capabilities.
Infusion Back into the Patient
Once enough CAR T-cells have been grown, they are infused back into the patient’s bloodstream, typically via a simple intravenous infusion. Often, the infusion takes place after the patient has received a lymphodepleting chemotherapy – killing off existing T-cells to create a ‘blank slate’ for the CAR T-cells – to help lower the number of other immune cells, giving the CAR T-cells greater ability to activate and fight the cancer. The infusion takes place in GW Hospital, where the patient will stay for approximately two to three weeks; the risk of cytokine release syndrome and neurotoxicity is high due to the expansion and cancer cell killing activity of CAR-T cells.
Targeting Cancer Cells
The CAR T-cells circulate throughout the patient's body, searching for and then binding to the specific antigen on the cancer cells’ surfaces. Once they attach to the cancer cells, the CAR T-cells activate and trigger a potent immune response. After binding to the cancer cells, the CAR T-cells continue to multiply and kill the cancer cells.
Of course, this is a very simplified explanation of how CAR T therapy works. It’s a complex process that requires precision and highly trained GMP-compliant lab technicians.
There are potential complications, as well: patients can experience Cytokine Release Syndrome (CRS). CRS occurs when cytokines, small proteins that control the growth and activity of blood cells and other immune system cells, are released into the body and can cause fever and chills, muscle aches and pains, exhaustion, and appetite loss. Another potential side effect is neurotoxicity – when the engineered T-cells produce an adverse effect on the nervous system’s functions, causing changes to the patient’s mental state, including confusion, difficulty with language or movement, and sometimes seizures. The medical team closely monitors the patient for any signs of CRS or neurotoxicity.
The promise of CAR T-cell therapy is bright. The field is still evolving, and ongoing research aims to expand the application to other cancers besides blood cancers and improve its safety and effectiveness. For now, it offers hope when hope may be out of reach and the potential of life without cancer.